Tubulin switches are controlled by nucleotide. GTP activates the protein upon polymerization and, GTPase activity take place within the microtubule lattice because only in the presence of axial contacts the nucleotide site is complete for hydrolysis. The chemical energy induces a mechanical strain that promotes microtubule disassembly in the absence of the GTP cap.
Structural studies are key to see proteins at atomic detail and to understand the molecular mechanisms involve in protein functions. In the case of tubulin these techniques have contribute to get snap shots of tubulin conformational changes along the polymerization-depolymerization cycle and related to the nucleotide bound state. Among those currently known are the curve-to-straight during assembly (favors by the formation of lateral contacts) and, compact-to-expanded during the GTPase cycle. Microtubule modulators function impeding the curve-to-straight change by wedging (within the tubulin heterodimer or between tubulin heterodimers) or the straight-to-curve by stabilizing the microtubule lattice. Some microtubule destabilizers simple block the filament tip comprising assembly.
We combine macromolecular crystallography of curved tubulin (currently we use tubulin-stathmin-tyrosine ligase and tubulin-darpin complexes) with X-ray fiber diffraction of polymerized microtubules to understand the effect of new natural products and synthetized compounds on tubulin conformation and microtubules structure. These techniques require synchrotron radiation and for these we have continue access to ALBA and ESRF facilities. Often, electron microscopy and cryo-electron microscopy contribute on the characterization of tubulin assemblies and, NMR spectroscopy allow us to decipher some particular features on the interaction of modulators with tubulin and microtubules.
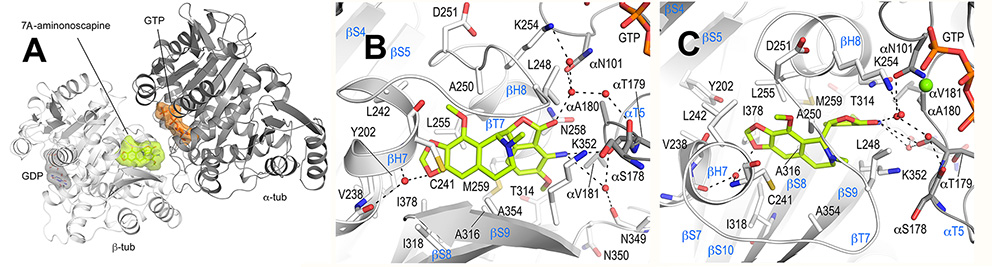
Figure- Overall tubulin-7A-aminonoscapine complex structure. A. Ribbon representation of the tubulin-bound 7A-aminonoscapine structure (PDB ID 6Y6D). The α- and β-tubulin chains are in dark and light grey, respectively. The ligand 7A-aminonoscapine (light green) and the nucleotides (orange) are in sphere and stick representation, respectively. The carbon atoms of the individual nucleotides are colored according to their chain assignments. Oxygen and nitrogen atoms are colored in red and blue, respectively. B, C. Close-up view of the atomic interaction network observed between 7A-aminonoscapine (light green) and tubulin (gray) in two different orientations. Interacting residues of tubulin are shown in stick representation and are labeled. Oxygen and nitrogen atoms are colored red and blue, respectively. Hydrogen bonds are depicted as black dashed lines. Secondary structural elements of tubulin are labeled in blue. For simplicity, only α-tubulin residues are indicated with a “α”.

