Pathogenic bacteria use macromolecular complexes, known as secretion systems, in order to transport toxic proteins into the medium, inject them directly into target cells or into other bacteria during niche colonization. In gram-negative bacteria, at least ten secretion systems have been described to date. Recently it has been described that the secretion system 6 (T6SS) machinery in strains of A. baumannii, including non-pathogenic strains, is functional and can act as effectively as that of V. cholerae in destroying E. coli cells. The core of the T6SS complex is composed of thirteen essential components (TssA to TssM). These nanomachine components are associated in three perfectly interconnected assemblies: a transmembrane complex (TssJLM), a membrane-associated basal complex (TssAEFGK) and a needle-like puncture structure (TssB, TssC, TssD (Hcp) and TssI (VgrG) with a PAAR protein at the end) that is propelled outward in a manner similar to the T4 bacteriophage virus. We have solved the structures for two components of the contractile tip, Hcp from A. baumannii and VgrG1 from P. aeruginosa, in addition to the soluble TssL (DotU) fraction of the transmembrane complex (TssJLM) and one of the proteins (TssK) of the membrane-associated basal complex (TssAEFGK).
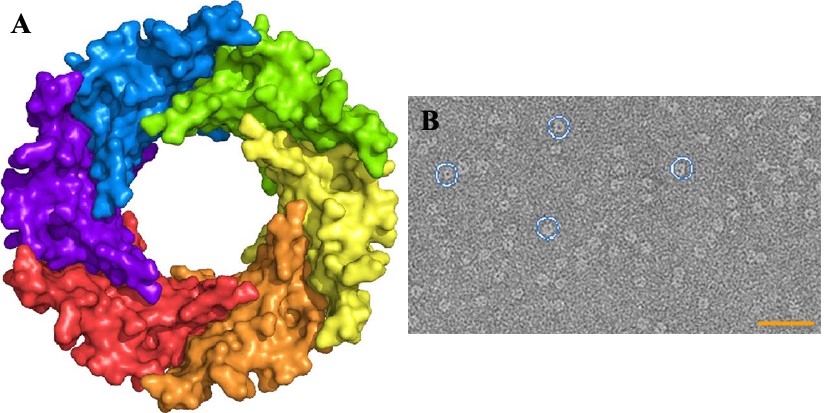
(A) 3D Structure of Hcp1 from A. baumannii (B) Electron microscopy photograph obtained by negative staining
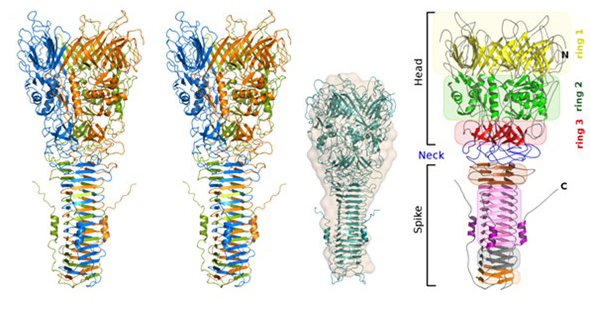
3D Structure of VgrG1 from P. aeruginosa
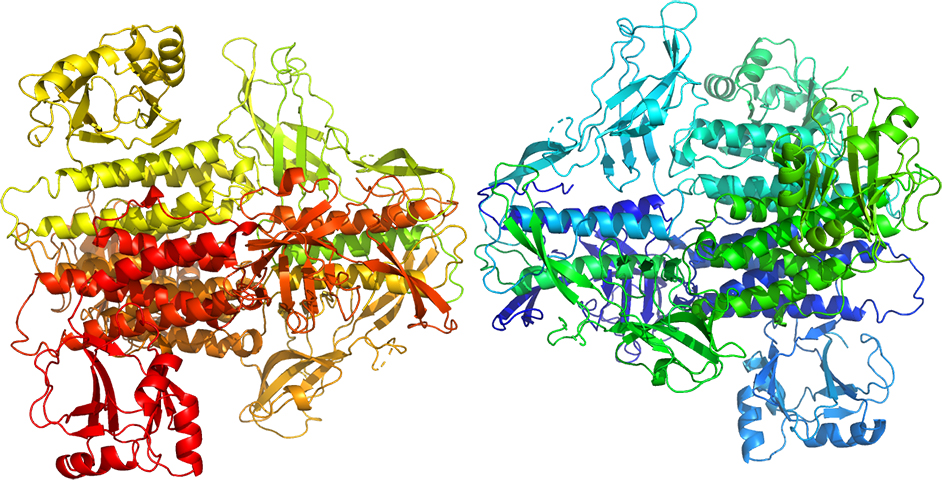
3D Structure of TssK from A. baumannii

