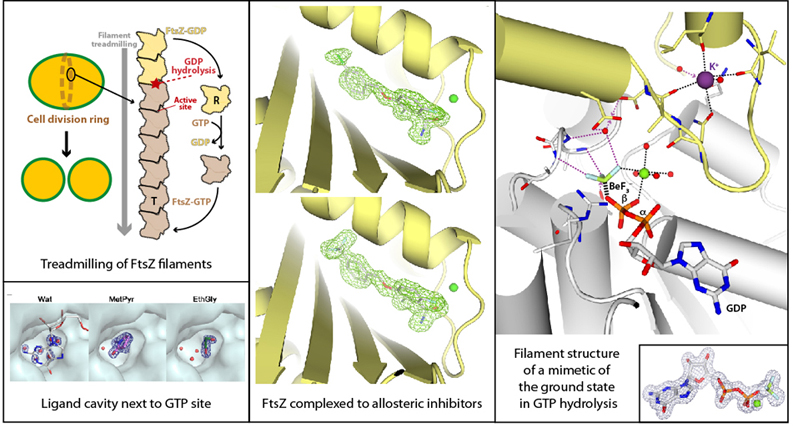
Cell division is an essential biological process that enables propagation of living organisms. In bacteria, the first step in cell division is the formation of a ring-like structure at the middle of the cell envelope, where the FtsZ protein plays a central role. FtsZ is an ancestral homologue of tubulin that polymerizes in the presence of GTP into polar filaments that treadmill upon GTP hydrolysis. Due to its essential role in bacterial cell division, inhibition of FtsZ is considered a suitable strategy to fight bacterial infections. We focused on the study of FtsZ from Staphylococcus aureus, an important human pathogen exhibiting resistance to available antibiotics.
We used X-ray crystallography and biochemical assays to study different folding states of FtsZ alone and in complex with small ligands, which revealed a cavity next to the nucleotide-binding pocket [FEBS J, 2020]. We also applied X-ray crystallography to determine structures of FtsZ in complex with novel inhibitors, which bind at an allosteric pocket and present bactericidal activity [J Med Chem, 2021]. Furthermore, we obtained high-resolution structures of FtsZ in complex with different nucleotide analogs and cations, including mimetics of the ground and transition states of catalysis, which shed light on mechanism of FtsZ filament dynamics [PLoS Biol, 2022]. Comparison with the structures of the FtsZ archaeal homolog allowed us to identify conserved GTPase active sites, likely inherited from the common ancestor [FEBS J, 2023].