MAMMARY STEM CELLS BIOLOGY AND THEIR RELATIONSHIP WITH MAMMARY CANCER STEM CELLS
The mammary gland undergoes most of its development after birth, and large remodeling and architectural changes occur with each reproductive cycle. In addition, it is known for over half a century that the mammary gland can be serially transplanted into recipient mice (1), indicating the presence of Mammary Stem Cells (MaSC) in this tissue.
Organ Size Control
Organ size control is a long-standing question in biology. In mammals, using conditional cell ablation, two mutually exclusive mechanisms involving either intrinsic or extrinsic programs have been described to control organ size. The mammary gland is an ideal model for such studies, since it undergoes size and morphological changes during puberty and pregnancy. The role of stem cells in controlling mammary epithelial tree size is unclear, although MaSC are able to reconstitute a functional organ upon transplantation. We have shown that mammary gland cellularity was strictly dependent on MaSC number, even following a 20-fold expansion of the mammary stem cell pool at puberty and transient 3-fold expansions with each pregnancy. In addition, the expansion of the MaSC pool was hormone dependent, as demonstrated by female bilateral ovariectomies during puberty and transplants of male-derived cells into female recipients. In these transplants, apart from a MaSC expansion, we also observed the donor cells reconstituting functional mammary glands, developing alveolar structures, and secreting milk after the recipient’s parturition. Taken together, these data suggest that in the mammary gland, there is a third organ size control mechanism, combining intrinsic cues throughout the organism’s lifetime, with extrinsic hormone signals at particular developmental windows (puberty, pregnancy), where an expansion of the MaSC pool occurs. This mechanism might have strong implications for the understanding of mammary tumorigenesis, since the expansion of the MSC pool precedes the generation of breast tumors (2).
Mammary Stem Cell (MaSC) expansion in response to physiological stimuli
The mammary stem cells (MaSC) in the mouse are CD24+CD29+ cells, and a single CD24+CD29+ cell is able, after transplantation to regenerate a functional mammary gland

Figure 1 Phase contrast micrograph of grown mammospheres, which can be micromanipulated and single mammospheres transplanted serially in primary, secondary, tertiary (not shown) and quaternary transplants, demonstrating that they contained at least one MaSC.
We determined that during puberty there is a 20-fold expansion on the MSC number, concomitant with a similar increase in the total epithelial cellularity. This was followed by a 3-fold transient expansion following each pregnancy cycle, with a retraction of the MaSC pool after involution (see Figure 2). The expansion during puberty is dependent on sexual hormones (estrogens and/or progesterone) since i) ovariectomy of the females at different times throughout puberty blocks MaSC expansion at the time of surgery, and ii) individuals lacking the appropriate concentration(s) of estrogens and progesterone (males) do not undergo MaSC expansion, although their MaSC are able to expand a generate a fully functional mammary gland when transplanted into recipients with the appropriate estrogen and progesterone concentrations (females) (see figure 3).
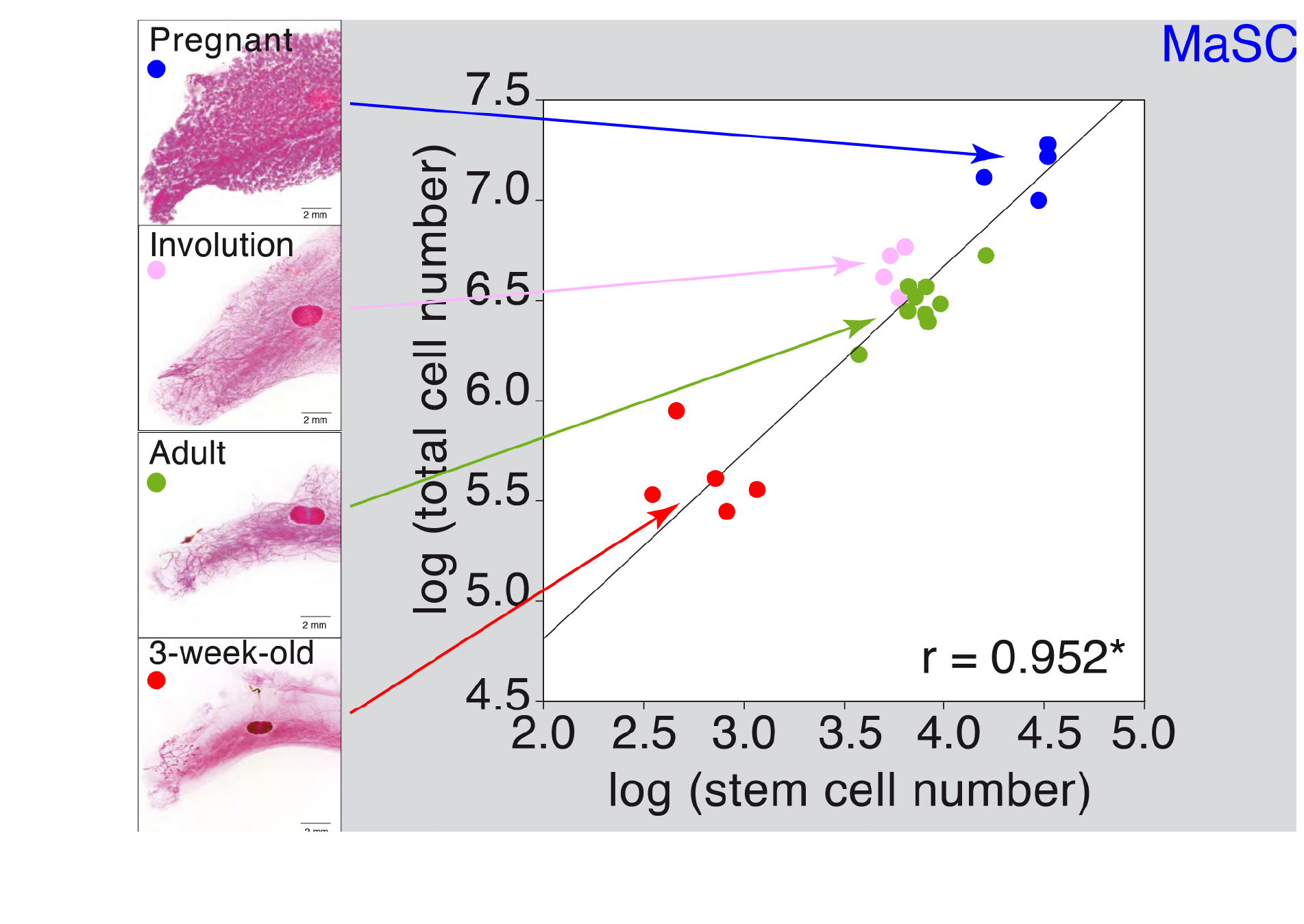
Figure 2 MaSC quantification during postnatal development and pregnancy. Mammary gland cellularity was plotted against stem cell number on the different postnatal and pregnancy stages.
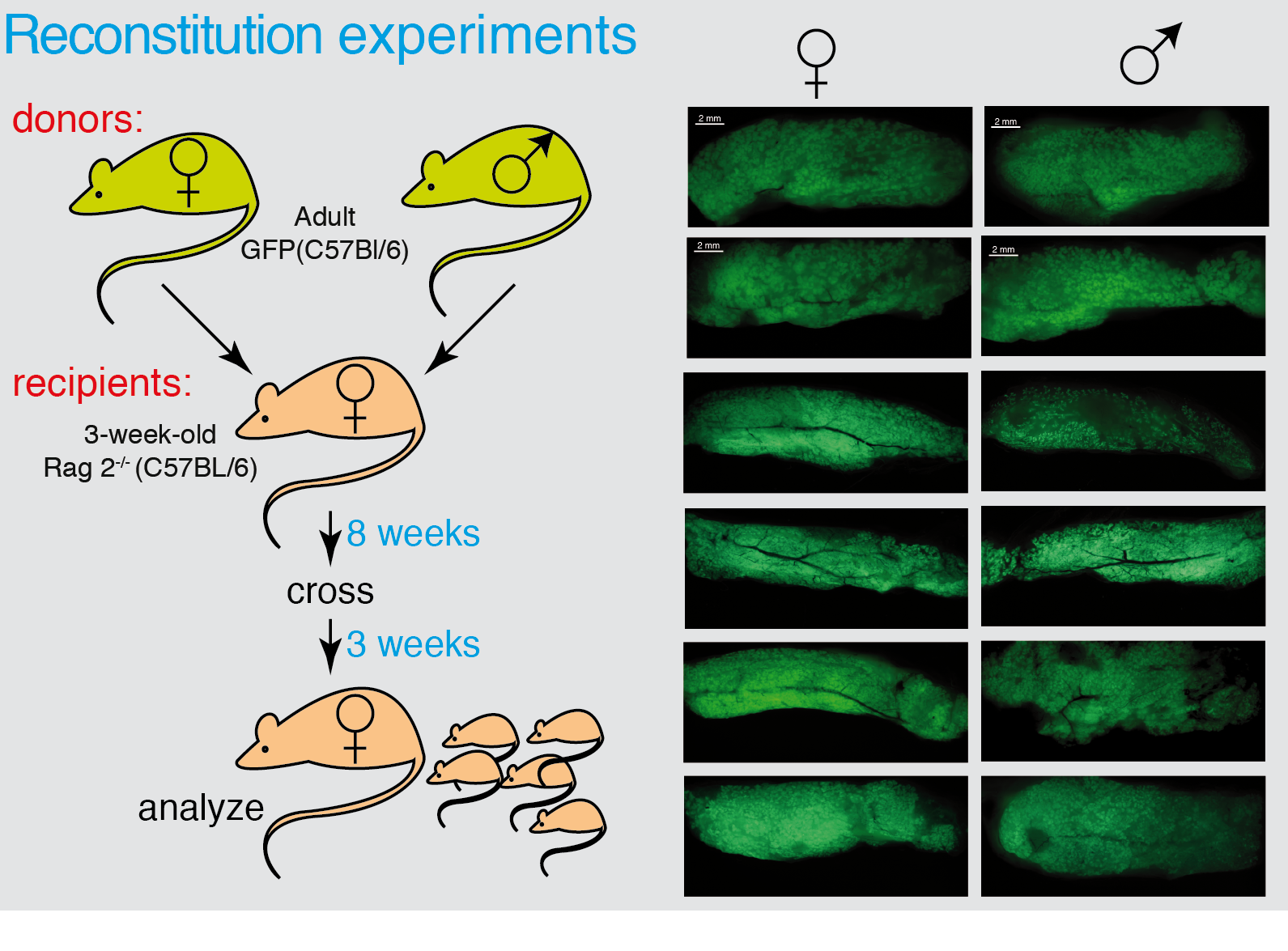
Figure 3 Male MaSCs are able to reconstitute a functional mammary gland. Whole mounts of male mammary glands transplanted with GFP-donor cells from female or male analyzed under UV light in a dissecting microscope.
Changes in stem cell pools have been described during embryonic development, following tissue damage or stress responses, but not for postnatal physiological responses
MaSC, organ size control and tumorigenicity:
The strict interdependence between stem cell number and total cellularity suggested that in the mammary gland, there is an intrinsic program controlling the size of the mammary epithelial tree similar to the one described for the pancreas
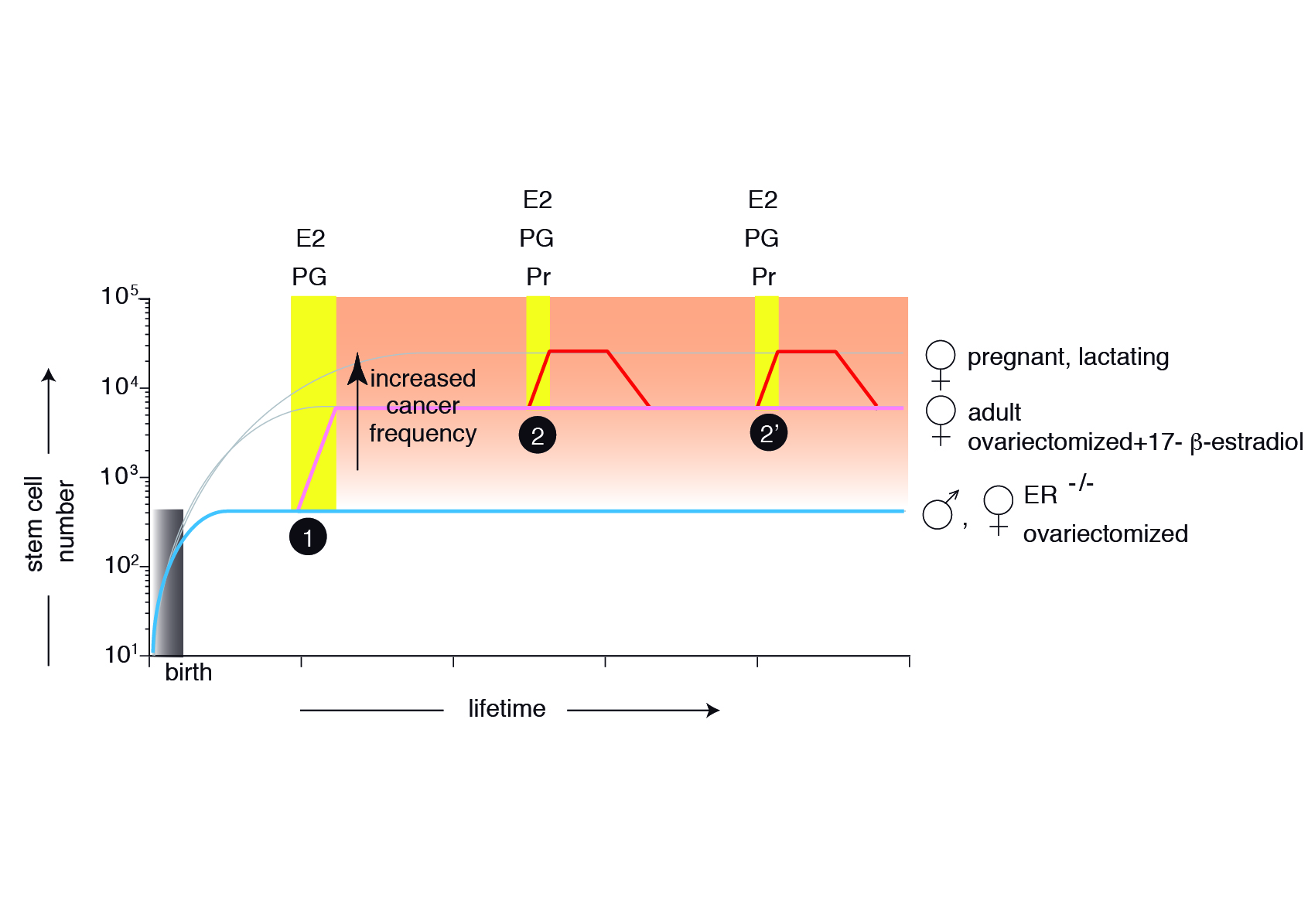
Figure 4 Model describing the mammary gland epithelial tree size control . The mammary epithelium is still rudimentary at birth, containing only a few MaSCs generated during embryonic development. In the absence of estrogens (E) and progesterone (Pg), the stem cell number will be maintained throughout the organism’s life (ER-/- females, ovariectomized females, males) (cyan). At puberty, hormones induce symmetric MaSC divisions, increasing 20-fold their number (pink). Hormone signals during each pregnancy will transiently increase an additional three-fold MaSC number (red), returning after involution to values similar to adult females. The changes during the estrous cycle are not depicted.
Our current efforts aim to verify in vivo the implications of this model on mammary tumorigenesis.
References
1. DeOme, K. B., Faulkin, L. J., Jr., Bern, H. A., and Blair, P. B. (1959) Development of mammary tumors from hyperplastic alveolar nodules transplanted into gland-free mammary fat pads of female C3H mice. Cancer Res 19, 515-520
2. Diaz-Guerra, E., Lillo, M. A., Santamaria, S., and Garcia-Sanz, J. A. (2012) Intrinsic cues and hormones control mouse mammary epithelial tree size. FASEB J 26, 3844-3853
3. Shackleton, M., Vaillant, F., Simpson, K. J., Stingl, J., Smyth, G. K., Asselin-Labat, M. L., Wu, L., Lindeman, G. J., and Visvader, J. E. (2006) Generation of a functional mammary gland from a single stem cell. Nature 439, 84-88
4. Stingl, J., Eirew, P., Ricketson, I., Shackleton, M., Vaillant, F., Choi, D., Li, H. I., and Eaves, C. J. (2006) Purification and unique properties of mammary epithelial stem cells. Nature 439, 993-997
5. Morrison, S. J., and Kimble, J. (2006) Asymmetric and symmetric stem-cell divisions in development and cancer. Nature 441, 1068-1074
6. Wilson, A., Laurenti, E., Oser, G., van der Wath, R. C., Blanco-Bose, W., Jaworski, M., Offner, S., Dunant, C. F., Eshkind, L., Bockamp, E., Lio, P., Macdonald, H. R., and Trumpp, A. (2008) Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell 135, 1118-1129
7. Stanger, B. Z., Tanaka, A. J., and Melton, D. A. (2007) Organ size is limited by the number of embryonic progenitor cells in the pancreas but not the liver. Nature 445, 886-891
8. Horner, M., Ries, L. A. G., Krapcho, M., Neyman, N., Aminou, R., Howlader, N., Altekruse, S. F., Feuer, E. J., Huang, L., Mariotto, A., Miller, B. A., Lewis, D. R., M.P., E., Stinchcomb, D. G., and Edwards, B. K. (2009) SEER Cancer Statistics Review, 1975-2006, National Cancer Institute. Bethesda, MD,. Vol. based on November 2008 SEER data submission, posted to the SEER web site, 2009. p. http://seer.cancer.gov/csr/1975_2006/, National Cancer Institute, Bethesda, MD
9. Asselin-Labat, M. L., Vaillant, F., Sheridan, J. M., Pal, B., Wu, D., Simpson, E. R., Yasuda, H., Smyth, G. K., Martin, T. J., Lindeman, G. J., and Visvader, J. E. (2010) Control of mammary stem cell function by steroid hormone signalling. Nature
10. Luetteke, N. C., Qiu, T. H., Fenton, S. E., Troyer, K. L., Riedel, R. F., Chang, A., and Lee, D. C. (1999) Targeted inactivation of the EGF and amphiregulin genes reveals distinct roles for EGF receptor ligands in mouse mammary gland development. Development 126, 2739-2750
11. Ciarloni, L., Mallepell, S., and Brisken, C. (2007) Amphiregulin is an essential mediator of estrogen receptor alpha function in mammary gland development. Proc Natl Acad Sci U S A 104, 5455-5460

