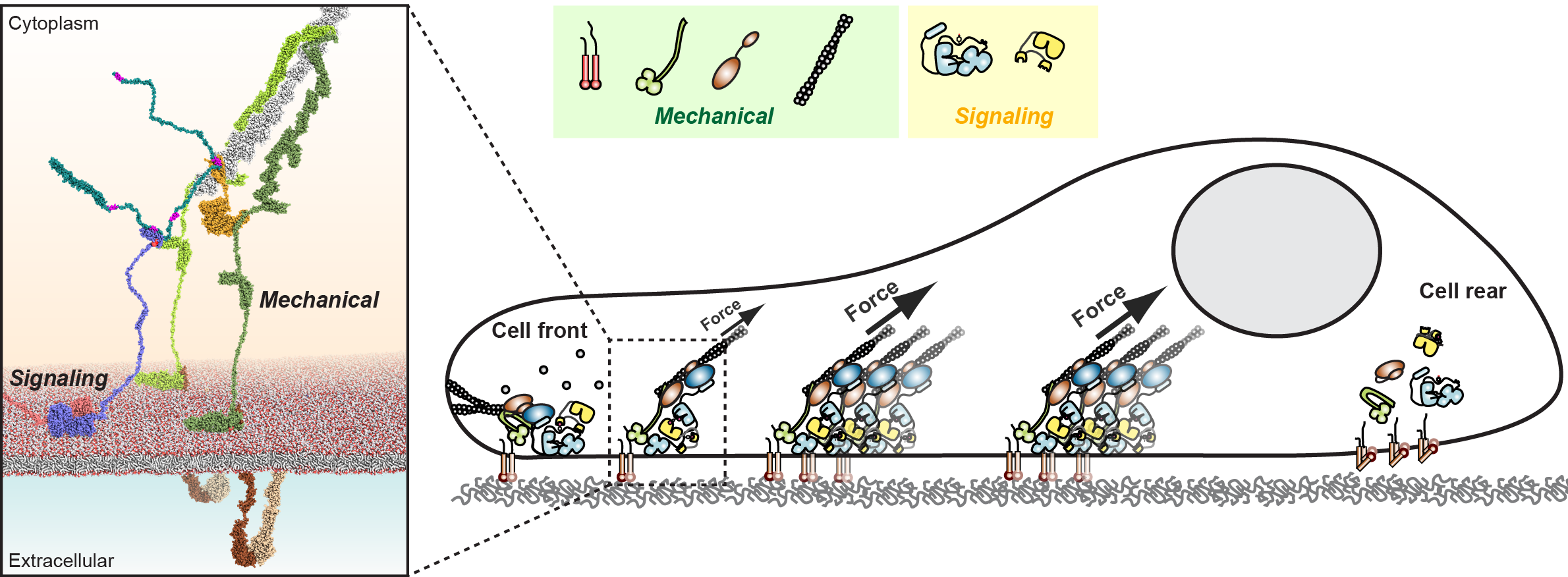
A work published in the journal Cell Commun. Signal. by the group led by Dr. Daniel Lietha at Centro de Investigaciones Biológicas Margarita Salas (CSIC) proposes an atomic model for Focal Adhesion Kinase (FAK) force activation that shows a phospho-regulated connection between signaling and force transduction components in focal adhesions (FAs), allowing for force-induced activation of FA signaling.
In higher organisms, cells have acquired the ability to migrate to specific locations in the body. In healthy organisms, coordinated cell movement is important during development or wound healing, and disease cancer cells can hijack this capability for cancer invasion and metastasis.
During cell migration, mechanical forces are generated, which on the one hand provide cell traction for movement and on the other hand activate an associated signaling apparatus. These signals are essential for coordinated and productive cell migration, controlling the formation of new adhesive traction points in the cell front and dissociation at the cell rear. Focal Adhesion Kinase (FAK) is a key signaling molecule in focal adhesions (FAs), orchestrating the formation, maturation, and turnover of the FA complex.
A long-standing question in the field has been how the mechanical force generated for cell migration is converted into biochemical signals. In this publication, the authors perform a multidisciplinary approach to answer this question.
Combining several biochemical, biophysical, structural, and cell biology techniques Díaz-Palacios et al. generated an atomic model of how proteins important for mechanical and signaling functions in cell migration can connect and explain how applied forces activate the signaling proteins. They have shown that the connection is highly regulated by a specific phosphorylation event and confirmed that both the connection and the phosphorylation are required to activate these signaling proteins in cells.
Understanding this mechanism at an atomic level will allow future design of new strategies to interfere with aberrant cell migration in cancer cells, thereby potentially providing new therapies to prevent cancer invasion and metastasis in advanced cancers.
The work has been funded by the Generation of Knowledge Grant PID2021-127058NB-I00 (D.L.) from the Spanish Ministry of Science and Innovation, co-funded by the European Regional Development Fund (FEDER).
Reference: Phospho-regulated tethering of focal adhesion kinase to vinculin links force transduction to focal adhesion signaling. Karen Diaz-Palacios, Pilar López Navajas, Bárbara Rodrigo Martín, Ruth Matesanz, Juan R. Luque-Ortega, Asier Echarri, and Daniel Lietha (2025) Cell Commun. Signal. 23:190 https://doi.org/10.1186/s12964-025-02201-3

