
A paper recently published in the journal Frontiers in Immunology by the group led by Prof. Vicente Larraga at the Margarita Salas Center for Biological Research (CIB-CSIC) presents the development of a DNA vaccine that fully protects mice against a lethal dose of SARS-CoV-2 virus. The results obtained in a murine model show that viral replication was controlled entirely in vaccinated mice's lungs, brain, and heart, making it a promising candidate for protection against COVID-19 disease.
The vaccine consists of a DNA plasmid, called pPAL, devoid of antibiotic resistance-based selection genes, and originally developed in Prof. Larraga's laboratory to obtain a vaccine against canine leishmaniasis.
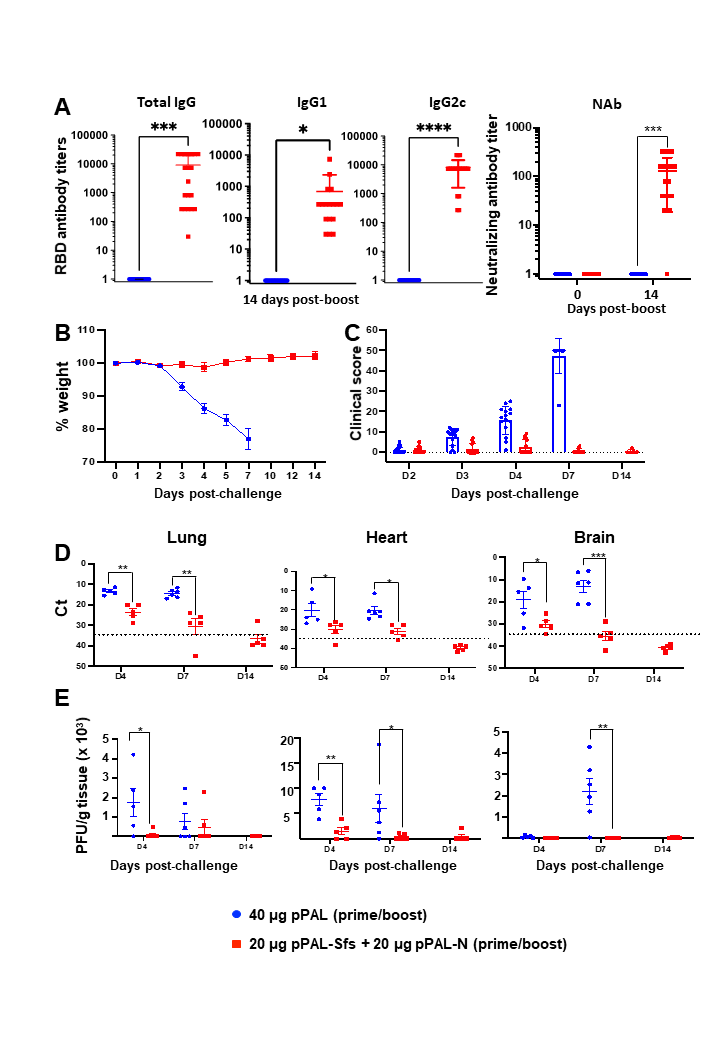
This plasmid has been adapted for the generation of a vaccine against SARS-CoV-2 with the introduction of the gene encoding the S-surface antigen of the virus, as well as the gene for the N-protein of the nucleocapsid. The final formulation is a mixture of both plasmids. While the surface protein has a high mutation frequency, the nucleocapsid protein is stable and contains T-cell activating sequences.
The vaccine developed by Alcolea et al. induces protein production in cultured human embryonic kidney HEK293 cells. Its administration in vivo, intramuscularly with electroporation in two doses, induces the production of high titers of specific antibodies that neutralize the SARS-CoV-2 virus. They also cause the activation of CD4+ and CD8+ cells. In protection tests, the vaccine candidate protects kh18 ACE2 mice, an animal model of COVID-19, against lethal doses (105 pfus) of both Wuhan strain 1 and Delta variant virus in a 100% of clinical signs of the disease.
The studies show that the treated animals show no signs of the disease during the test (14 days) in contrast to the unvaccinated controls, which lose weight from day two and die on day seven. In addition, the vaccine induces a significant reduction of the viral load in the target organs of the disease (lung, heart, and brain), not only in its detection by PCR but also as an active virus in vitro.
Overall, the results demonstrate that this vaccine induces very high protection in a murine model, and has also passed toxicity tests in dogs. Additionally, it is thermotolerant, which would allow its distribution in countries where maintaining the cold chain is difficult, and it is being tested for subcutaneous administration with a "needle-free" procedure. These factors make this candidate a promising vaccine against COVID-19.
Reference: Non-replicative antibiotic resistance-free DNA vaccine encoding S and N proteins induces full protection in mice against SARS-CoV-2. Pedro J. Alcolea, Jaime Larraga, Daniel Rodríguez-Martín, Ana Alonso, Francisco J. Loayza, José M. Rojas, Silvia Ruiz-García, Andrés Louloudes-Lázaro, Ana B. Carlón, Pedro J. Sánchez-Cordón, Pablo Nogales-Altozano, Natalia Redondo, Miguel Manzano, Daniel Lozano, Jesús Palomero, María Montoya, María Vallet-Regí, Verónica Martín, Noemí Sevilla and Vicente Larraga (2022) Frontiers in immunology. 13:1023255. DOI: 10.3389/fimmu.2022.1023255

